Single cell analysis is a new trend that makes it possible to discover mechanisms not seen when studying a bulk population of cells. Our label-free techniques are capable of measuring single cell adhesion force and other properties, and deposite single cells as well. The CellSorter automatized micropipette system is capable of sorting single cells with great accuracy and speed. Fluid FM is a truly unique combination of force microscopy and microfluidics elevates applications to a higher level, from single cell biology to surface analysis and beyond.
Collaboration partners: CellSorter¸ Eötvös Lóránd University

Cells prefer to adhere onto the RGD displaying surfaces printed by FluidFM.
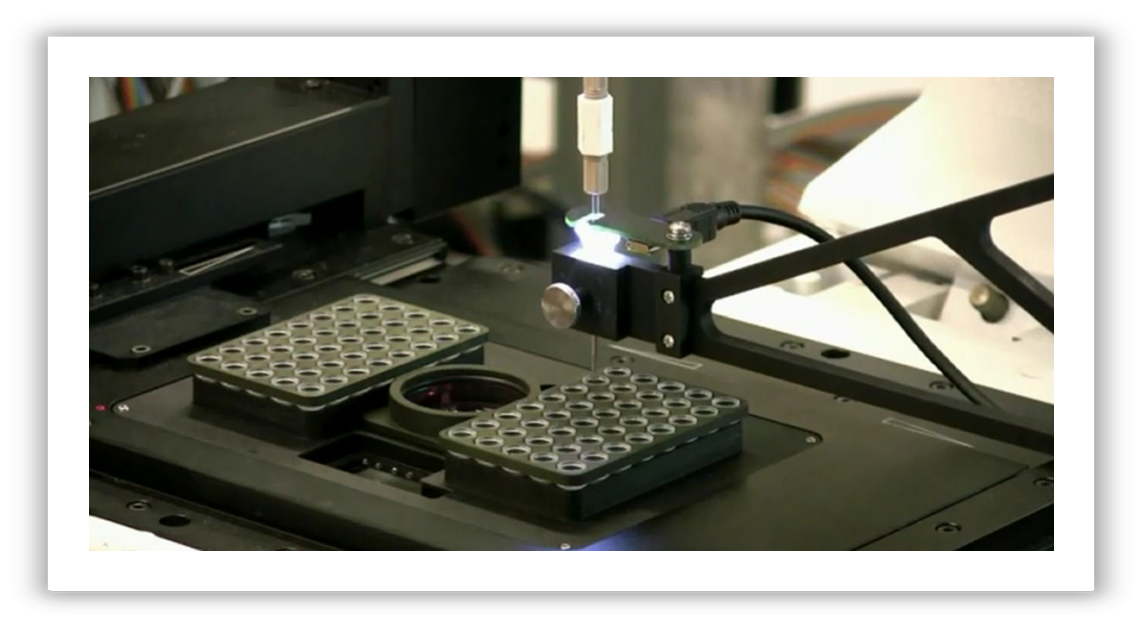
Cells are deposited from the micropipette either onto a glass cover slip or into PCR tubes, both fixed in the insert.
Single cell transfer is carried out by moving the motorized stage horizontally back and forth between
the Petri dish and the PCR tubes (or the cover glass).
Single cell transfer is carried out by moving the motorized stage horizontally back and forth between
the Petri dish and the PCR tubes (or the cover glass).
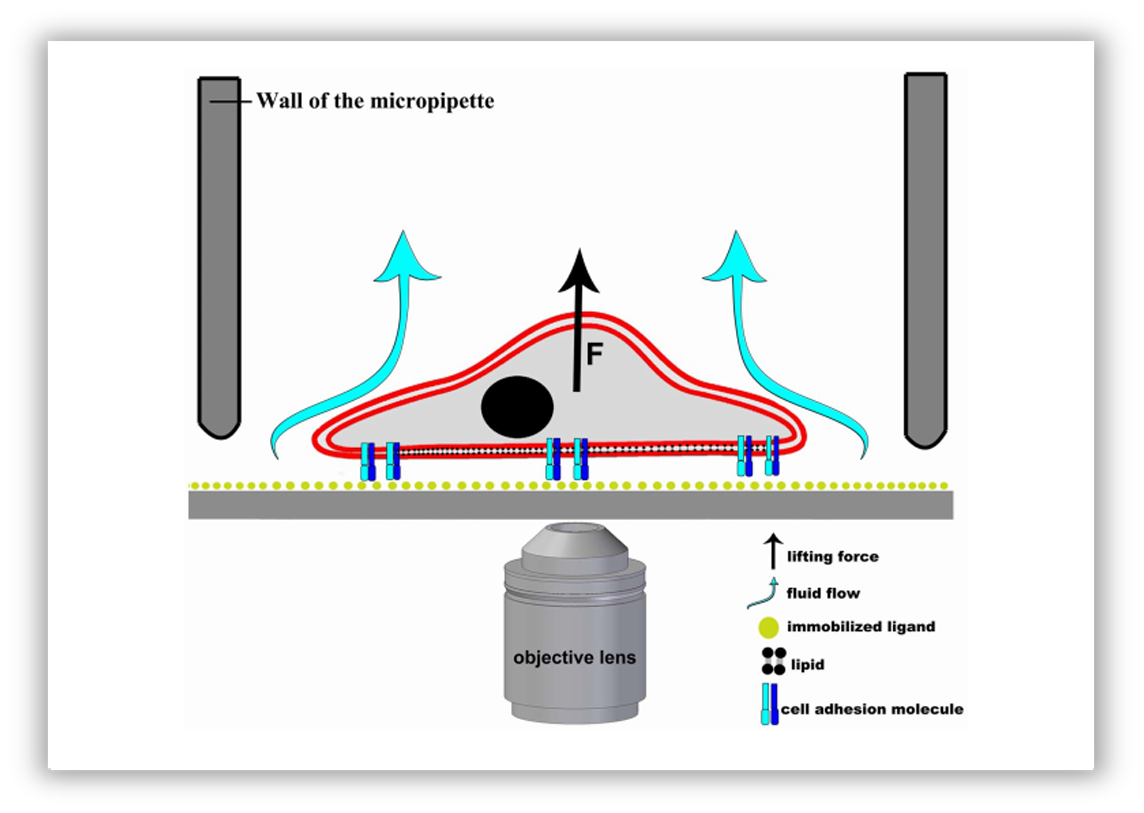
Schematic representation of the hydrodynamic adhesion force measurement on a single cell using a micropipette.
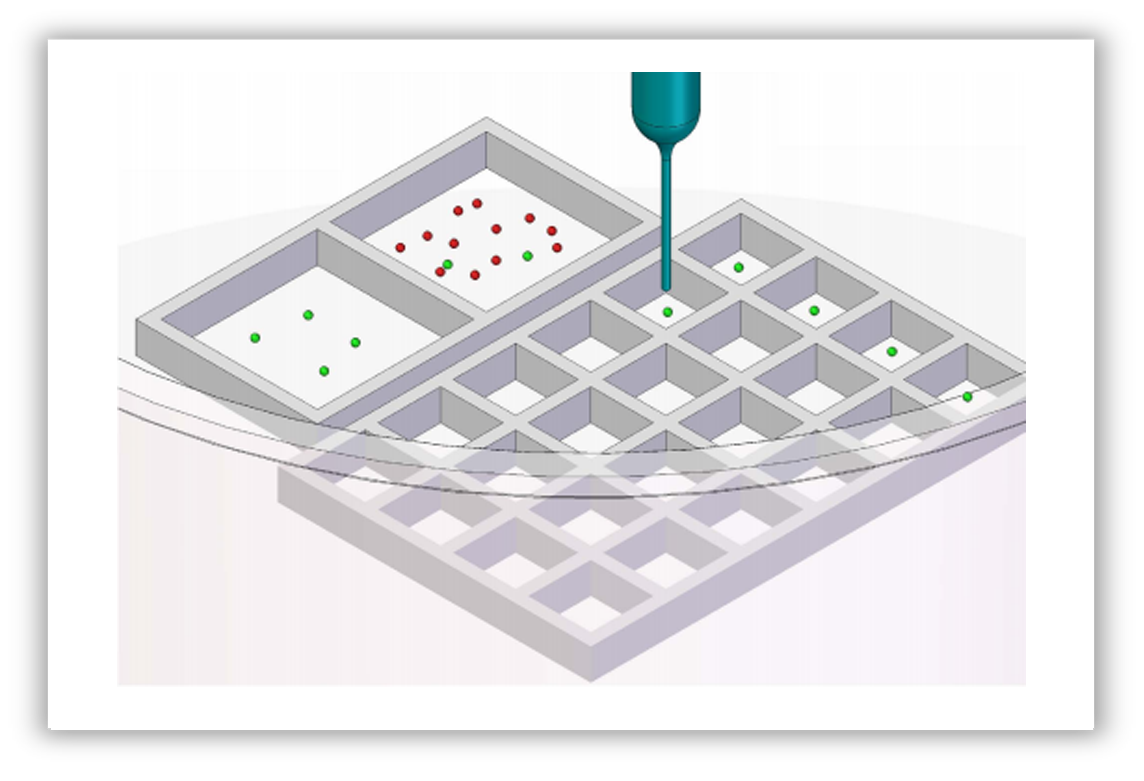
Automated micropipette for single cell isolation; initial suspension was kept in a larger square.
Green and red dots represent cells to be isolated and cells not needed, respectively.
Selected single cells could be directly moved from this square to the other large
square or into smaller squares in the same dish or into PCR tubes.
Green and red dots represent cells to be isolated and cells not needed, respectively.
Selected single cells could be directly moved from this square to the other large
square or into smaller squares in the same dish or into PCR tubes.
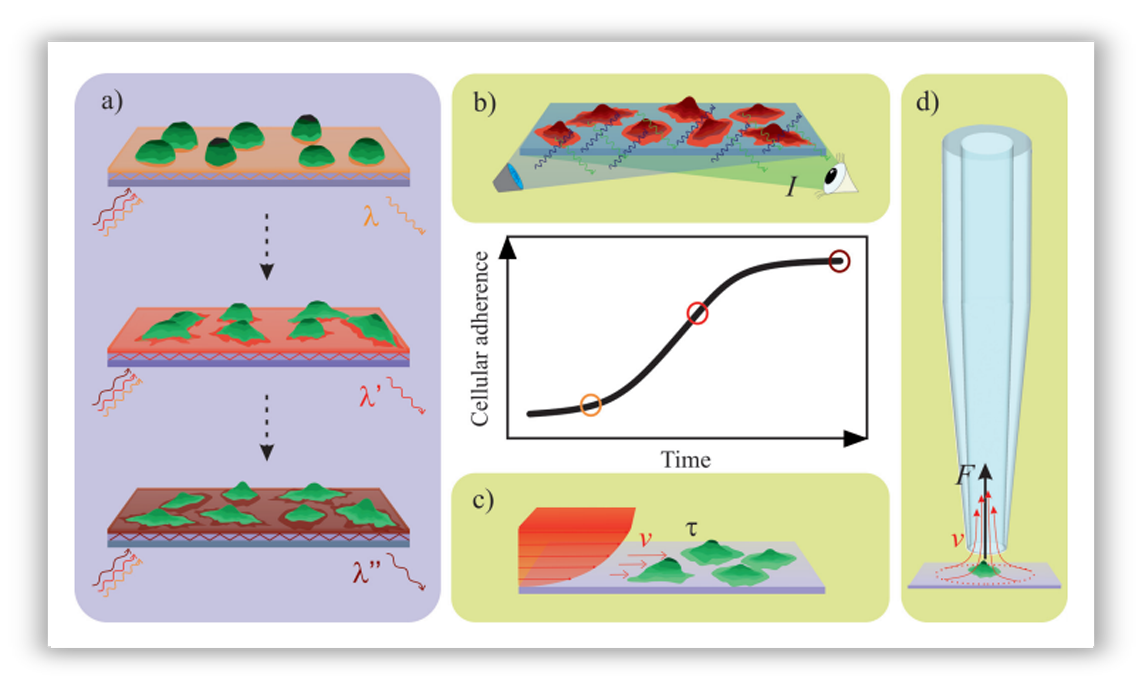
Schematic illustration of the working principle of the techniques employed to characterize immune cell adherence.
(a) The detection principle of the high-throughput optical biosensor. (b) Adherence measurement in a classical
(a) The detection principle of the high-throughput optical biosensor. (b) Adherence measurement in a classical
end-point assay; cells are labeled with a fluorescent dye. (c) Adherence measurement in a flow chamber
(rectangular microfluidic channel). (d) Adherence measurement with the automated micropipette.
(rectangular microfluidic channel). (d) Adherence measurement with the automated micropipette.
Relevant publications:
Salanki et al. Single Cell Adhesion Assay Using Computer Controlled Micropipette. Plos One, 2014
Salanki et al. Automated single cell sorting and deposition in submicroliter drops. Applied Physics Letters, 2014
Sandor et al. CD11c/CD18 Dominates Adhesion of Human Monocytes, Macrophages and Dendritic Cells over CD11b/CD18. Plos One, 2016
Ungai-Salanki et al. Automated single cell isolation from suspension with computer vision. Scientific Reports, 2016
Orgovan et al. Adhesion kinetics of human primary monocytes, dendritic cells, and macrophages: Dynamic cell adhesion measurements with a label-free optical biosensor and their comparison with end-point assays. Biointerphases, 2016

